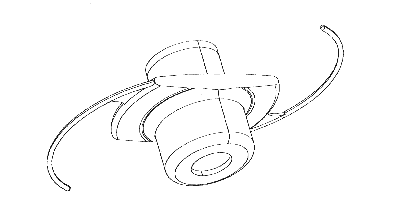
Fig. 1. The Miniaturized Telescope mounted in a carrier intraocular lens with haptic loops.
Implantable Miniaturized Telescope (IMT) for Low-Vision
Eli Peli 1, 2, Isaac Lipshitz 2, and Gideon Dotan 2
1
The Schepens Eye Research Institute, Harvard Medical School, Boston MA, USA2
VisionCare, Inc., Yehud, IsraelAbstract
VisionCare has developed and tested the first Implantable Miniaturized Telescope (IMT) for low vision. A small optical device with a configuration of a Galilean telescope is implanted in place of the crystalline lens. The IMT is inserted and held in position using a surgical procedure similar to a standard intraocular lens. Together with the cornea, the IMT acts as a telephoto lens that is
in focus at a distance of 50 cm. For closer and further distance views, eyeglasses are needed. A nominal magnification of 3.05 is achieved with the IMT. Even though higher relative distance magnification power may be provided with additional low diopter eyeglasses, distance vision may be provided also with negative lenses in the spectacles. The main advantage of the IMT over a spectacle or head-mounted low-vision telescope is the flexibility afforded by the ability to scan reading materials and other images using natural eye scanning movements. The fact that the magnification is in the eye completely eliminates the increased speed of motion and vestibular conflict, which impedes the use of other head-mounted low-vision telescopes. The IMT is designed for monocular use in patients with bilateral acuity loss due to macular diseases and other retinal pathologies. Prototype telescopes were built and tested to meet the specifications for 3.05 magnification, a 6.6º visual field (20º on retina), and excellent optical quality. The effective field of view of the IMT is much wider than an equivalent head mounted telescope, as scanning with eye movements only is required. Preliminary results of clinical trials are reported here.
Introduction
Low vision or visual impairment affects mostly the elderly. With the rapid aging of the population distribution in the USA and other industrialized nations, both the numbers and proportions of people with visual impairment are expected to increase rapidly in the next two decades. The most common cause of vision disability is age related macular degeneration (ARMD), which affects the fovea, the central section of the retina used for high-resolution vision (Klaver et al., 1998). Prevent Blindness America (1994) reported about 3.5 million Americans over the age of 40 years have moderate to severe vision loss including blindness (about 300,000). About half of these impairments are due to ARMD. Like ARMD, diabetic retinopathy, optic neuropathy, central retinal vein occlusions and other conditions also cause central field loss (CFL). CFL reduces the patient’s resolution and affects their ability to conduct activities of daily living such as reading, recognizing faces, watching television and driving.
Normally when reading, people move their eyes rapidly across the text scanning about 5 words per second, leading to a reading rate of as much as 300 words per minute, However, with CFL reading rates drop below 100 words per minute. A variety of magnifying devices have been use to aid patients with CFL read. Most magnifying devices have a restricted field of view and have to be manually scanned across the text, magnifying a small portion at a time. In some cases the text is scanned in front of the magnifying device and held at a very short, fixed viewing distance. In all cases the manual scanning is slow and limits the reading rate.
Spectacle mounted telescopes have been used as low vision aids for about 50 years. The magnification provided by the telescope effectively compensates for the loss of resolution suffered by patients with CFL. Thus objects seen through the telescopes may be recognized from distances at which they will not be recognized with unaided vision. Near vision telescopes provide magnification for reading at a more comfortable distance than microscopic spectacles. However, the field of view through the typical low vision telescope is narrow (6
º to 12º for 85 to 35 telescopes, respectively). With such a narrow field, navigation in the visual environment is difficult (and may be dangerous) and requires scanning head movements rather than the natural eye movements. In addition, the magnified visual motion of the environment seen through the telescope conflicts with the vestibular head movement information from the inner ear, causing difficulties in adaptation to devices when worn centrally in the spectacle lens and used continuously (Demer et al., 1989). Thus, although low vision telescopes are occasionally used in the central position for reading tasks, the most successful application of this technology is for distance vision and used as a bioptic.The bioptic telescope is mounted at the top of the spectacle lens, above the pupil, with a slight inclination upwards. Most of the time, the patient views the environment through the regular spectacle lens (the carrier lens) enjoying the benefits of intact peripheral vision. When a distant object is detected and cannot be recognized due to reduced resolution (CFL), the patient tips his head slightly down, bringing the telescope in front of the eye and the object of interest into the field of view of the telescope. A short examination (1 to 2 sec) of the target through the telescope provides the patient with the required high detail information needed to recognize the target. This intermittent use makes the bioptic telescope an effective, comfortable, and safe device. Despite some adverse opinions (Fonda, 1983), low vision telescopes are permitted as visual aids for driving in 27 states in the USA (Peli and Pely, 1999).
The use of a single bioptic telescope by a patient with two functional eyes represents another improvement. When the patient views through the telescope, the magnification of the telescope creates a ring scotoma or field loss. If a 10
º view is visible through a 4.05 telescope, it occupies a retinal area of 40º causing a ring of 15º of the surrounding environment to be obscured. However, when only a single telescope is used, the fellow eye continues to see that part of the environment that is lost to the eye with the telescope. This is an important safety feature, since if a threat or obstacle appears at that field location during the telescopic glimpse it will be detectable by a patient with a single telescope, but not by a patient with binocular bioptic telescopes.Despite all these advantages, spectacle and head mounted telescopes have gained limited use by patients with CFL as reading or distance vision aids. The reasons for the limited acceptance of the devices are presumed to be: the obvious and unattractive appearance of the devices (Spitzberg et al., 1989), the need to use slow head scanning movements in place of the eye movements used in natural vision, and the vestibular conflict resulting from the increased motion which accompanies head-mounted magnification (Demer et al., 1989). The latter effect may cause a conflict between the visual and vestibular system leading to discomfort or motion sickness. While the vestibular system can adapt easily to low levels of magnification (i.e. a few percent) as created by spectacle lenses, the ability to adapt to the large magnification needed in low vision telescope have not been demonstrated.
In this article we describe the development and preliminary clinical results of an effort to develop an implantable telescopic lens. With the telescopic lens inside the eye, we get an eye which performs as a telephoto lens with a focal length three times the focal length of the normal eye. In this case, most of the limitations of the head-mounted telescopes are avoided. We believe this should lead to a successful use of the telescope as both a reading and a distance vision aid.
The Implantable Miniaturized Telescope (IMT)
As shown in Fig. 1, the IMT is a miniature Galilean telescope mounted on a PMMA carrier intraocular lens implant (Lipshitz et al., 1997). The IMT is implanted in the posterior chamber of the eye, in place of the crystalline lens. It is held in position in the lens capsule by haptic loops and the IMT bulges forward into the anterior chamber through the pupil (Fig. 2). The IMT is implanted using a modified cataract extraction procedure. It is designed to be implanted in one eye of a patient with symmetrical CFL. With no additional lens, the IMT should provide 3.05 magnification at a distance of 50 cm, providing better resolution for many activities of daily living. With the use of additional spectacle lenses, we can focus at any distance from 25 cm to infinity. With the use of an additional lens for reading at shorter distances, the relative effective magnification can be increased, up to 85 . Also, the IMT can be used for distance vision with the required refractive correction in the spectacles (-2.00D).

Fig. 1. The Miniaturized Telescope mounted in a carrier intraocular lens with haptic loops.
Patients with refractive error prior to this surgery will need similar correction to their pre-surgical correction to achieve the same effect. The useful field of view through the telescope is limited to 6.6º (with a wider extent of field available at low resolution and contrast). For this reason the telescope is implanted in one eye only to provide high-resolution central vision, while the fellow eye continues to be used for peripheral vision and safe mobility. With the telescope, vision is expected to improve sufficiently to provide comfortable reading at a conventional distance. While the instantaneous field of view is small, the ability to scan using eye movements should provide much more comfortable reading than would be possible with a head mounted telescope of the same field.
The IMT is constructed from two glass lenses inside a glass tube. Two flat glass windows keep the lenses separated from the aqueous humor, increasing the effective power of the lenses relative to lenses immersed in the aqueous. Also the airtight tube serves to float the telescope in the eye, so that its effective weight is only 46 mg. The tube is 3.0 mm in diameter and 4.4 mm long. The front of the IMT is positioned about 2 mm behind the posterior surface of the cornea. The iris is used to support and center the IMT inside the eye and on the optical axis, and helps block non-direct light from reaching the retina, and so increases the contrast.
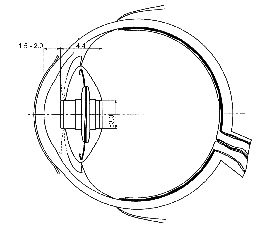
Fig. 2. The IMT mounted in the lens capsule and supported by the iris
Testing of the telescope in a schematic eye was conducted to determine the performance of the telescope. Along its axis, the overall modulation transfer function (MTF) of the system (cornea + IMT) was found to be as high as 0.95 at 8 lp/mm (1 c/deg is equal to about 3 line-pairs per millimeter (lp/mm) on the retina). Thus the MTF at 2.7 c/deg on the retina is very high. Considering the 35 magnification of the telescope this is equivalent to 8 c/deg in object space. A patient with 20/200 acuity can read letters of 50 arc minutes (or 0.8º) or details of 2.5/0.8 = 3.1 c/deg. Thus the contrast through the telescope at the resolution limit of such patients is sufficient. The required magnification for fluent reading of newspaper size text for such patients however will be closer to 6
º than 3º. Therefore, reading at a shorter yet comfortable distance (25 cm) will be required.The advantages of implanted telescope over head mounted telescope
Using any head mounted magnifying device causes a disruption of image stability and perceived direction as a result of head motion (Miles, 1991). In natural viewing without optical devices, if an observer fixates a target at primary position of gaze then rotates his head, while maintaining fixation on the same target, the required change in eye position in the orbit is equal in magnitude and opposite in direction to the head rotation (Fig. 3a). Such an eye movement is generated automatically by the vestibular ocular reflex (VOR) in response to head movements and it serves to maintain a stable retinal image during head rotation. With the use of a head mounted magnifying device, the required correcting eye movement is larger, as illustrated in Fig. 3b. When using a telescopic device with a 3.05 magnification, an eye movement three times as large as the head rotation is required. While the ability of the VOR system to adapt to large changes in magnification (up to 35 %) that may occur (e.g. with diving goggles) have been demonstrated (Gauthier and Robinson, 1975), the ability to adapt to the extreme demand of this telescope (300% magnification) has not been demonstrated. Furthermore, it should be noted that when using a monocular telescope, as is commonly the case, the demand for adaptation is different between the two eyes (Fig. 3b). Some capacity to adapt the VOR of each eye differentially has been demonstrated in monkeys (Viirre et al 1987; Snow et al 1985), but again only small changes in VOR were demonstrated. If the VOR cannot adapt differentially between the eyes, every head movement with a head-mounted magnifying device will result in substantial image motion in one eye with the accompanying reduction in sensitivity (Peli et al., 1998). However, using the IMT resolves this problem completely. As illustrated in Fig. 3c, with the IMT a given head movement will require a compensatory eye movement of the same magnitude. Thus the natural VOR gain of about 1.0 will suffice. In addition, no conflict will occur between the image motions in both eyes with a monocular IMT.
While the discussion above was framed in terms of head movement it should be clear that the same arguments apply when the observer shifts fixation between two targets without any eye movements. With the IMT, a target 5º from fixation will require only 5º of eye movement to be examined with an IMT but will require 15º of eye movement with a head mounted telescope. Further, if a target is seen with the other eye to be at a certain angular distance from fixation it may be acquired through the IMT with the corresponding size of eye movement. Thus the IMT offers a distinct advantage in space and direction perception as well as clarity of vision in both eyes during eye movements, head movements and tracking of moving targets.
Clinical Trials
Clinical trials are being conducted in Europe. We report here the results of the first 24 patients implanted with the IMT and analyzed by March 31, 1999. Initial clinical trials were conducted in nine blind eyes to test the mechanical feasibility of the implant procedure. The first seven of these were operated by Open-Sky technique. The later procedures were modified to reduce the impact of the surgery on the cornea. A corneal incision of 140º was used to remove the lens using phaco-emulsification. The IMT was inserted through the incision and was placed into the bag with the help of a second tool.
Patient selection in the early trials was limited to patients with bilateral dry-type ARMD of about equal acuity in both eyes, not better than 20/100 (6/30). Follow-up of more than one month was available on 12 patients at the time of writing. Average follow-up for these patients was 2.2 months (range 1 to 6 months). All of these patients had bilateral CFL due to ARMD. The average age of the patients was 75.3 years (range 65 to 83 years). Most patients reported an improvement in visual function following a few weeks of adaptation. Distance VA through the telescopes was improved in 10 of the 12 patients. The group mean VA was better post-operatively and the difference was statistically significant. Functional improvement was reported by most patients for a number of tasks: watching television – 91%, table orientation – 75%, and reading – 66%. All these changes were statistically significant.
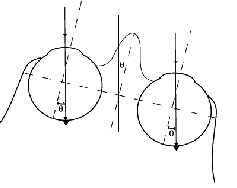
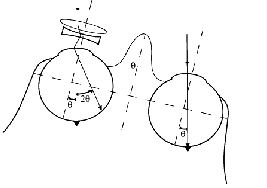
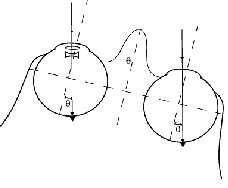
Fig. 3.
The advantages of a monocular intra-ocular telescope, in terms of image stability and
stable directions of gaze, are illustrated.
(a) To maintain fixation on an object in natural viewing
(i.e. without an optical device) a head rotation of a given angle (T deg.)
requires a compensatory
eye rotation of the same angle but in the opposite direction (-T deg.).
The vestibular system, in the
inner ear, will assist such an eye rotation through the vestibular ocular reflex (VOR). (b) With
a head-mounted telescope, due to the magnification (i.e. 3T deg.), the VOR generated eye rotation (-T deg.)
will not suffice to maintain fixation with that eye. This causes perceived image motion,
reduced visibility, and possibly motion sickness symptoms. (c) When the telescope is inside
the eye, the VOR generated eye rotation will restore fixation in the same way it does for the
unaided eye. Note also, that with the head-mounted monocular telescope, the eye movements
required from both eyes differ, while they remain equal for the monocular intra-ocular
Conclusion An implantable miniaturized telescopic device for low vision was developed and tested. Preliminary results are encouraging as the implant procedure is feasible and no serious complications were encountered. It is even more encouraging to find that the implant functions to a large extent as it was designed, providing useful magnification and functional vision. The use of two eyes with widely differing magnifications and fields of view will be a challenge for the patients. Understanding this situation and the ways patients can adapt to it effectively is the biggest challenge facing this project. If successful, the IMT will represent a new treatment option for patients with CFL and moderate loss of acuity that might be superior to all existing devices. Patients with CFL represent the majority of people affected by ARMD and low vision in general. References Demer, J.L., Porter, F.I., Goldberg, J., Jenkins, H.A. and Schmidt, K. (1989). Adaptation to telescopic spectacles: Vestibulo-ocular reflex plasticity. Investigative Ophthalmology and Visual Science, 30, 159-170. Fonda, G. (1983). Bioptic telescopic spectacle is a hazard for operating a motor vehicle. Archives of Ophthalmology, 101, 1907-1908. Gauthier, G.M. and Robinson, D.A. (1975). Adaptation of the human vestibular ocular reflex to magnifying lenses. Brain Research, 92, 331-335. Klaver, C.C.W., Wolfs, R.C.W., Vingerling, J.R., Hofman, A. and de Jong, P.T.V.M. (1998). Age-specific prevalence and causes of blindness and visual impairment in an older population: the Rotterdam Study. Archives of Ophthalmology, 116, 653-658. Lipshitz I., Loewenstein A., Reingerwirtz M., and Lazar M. (1997) An intraocular telescopic lens for macular degeneration. Ophthalmic Surgery and Lasers, 28, 513-517. Miles F.A. (1991). The disruptive effects of optical aids on retinal image stability during head movements. In Presbyopia Research: From Molecular Biology to Visual Adaptation, Obrect G. and Stark L.W. eds., Plenum Press, New York 113-125. Peli, E., Fine, E. and Labianca, A. (1998). The detection of moving features on a display: The interaction of direction of motion, orientation, and display rate. Technical Digest of Papers, SID-98, Digest, 1033-1036. Peli, E. and Pely, D. (1999). Driving with Low Vision. Doing It Your Way. How to Preserve, Maintain and Extend Your Driving Skills and Privileges, Pamphlet in press. Prevent Blindness America. (1994). Vision Problems in the U.S. Schaumburg, Illinois: National Society to Prevent Blindness. Snow, R., Hore, J. and Villis, T. (1985). Adaptation of saccadic and vestibulo-ocular systems after extraocular muscle tenectomy. Investigative Ophthalmology and Visual Science, 26, 924-931. Spitzberg, L.A., Jose, R.T., Kuether, C.L. (1989). Behind the lens telescope: A new concept in bioptics. Optometry and Vision Science, 66, 616-620. Viirre, E., Cadera, W. and Vilis, T. (1987). The pattern of changes produced in the saccadic system and vestibuloocular reflex by visually patching one eye. Journal of Neurophysiology, 57, 92-103.